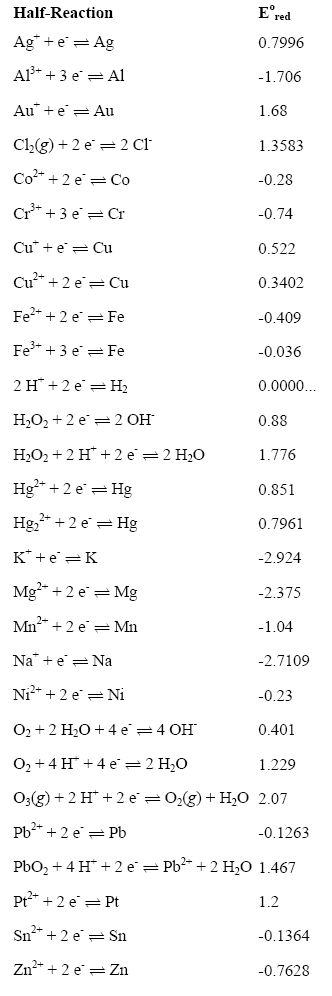Table 2 from The standard electrode potential (Eθ) predicts the prooxidant activity and the acute toxicity of metal ions. | Semantic Scholar
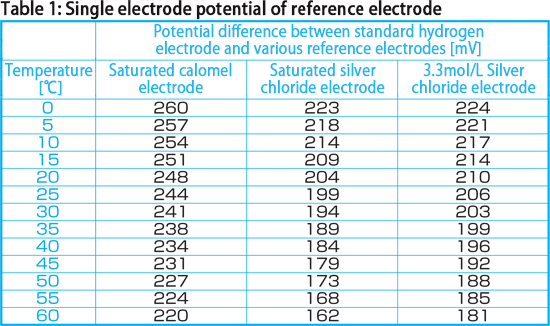
Single electrode potential of reference electrode | Useful information | Product Support | DKK-TOA CORPORATION
![PDF] Standard Electrode Potentials and Temperature Coefficients in Water at 298.15 K | Semantic Scholar PDF] Standard Electrode Potentials and Temperature Coefficients in Water at 298.15 K | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/56964684a624c5af38c7e62256db3faa4c542d88/19-Table2-1.png)
PDF] Standard Electrode Potentials and Temperature Coefficients in Water at 298.15 K | Semantic Scholar

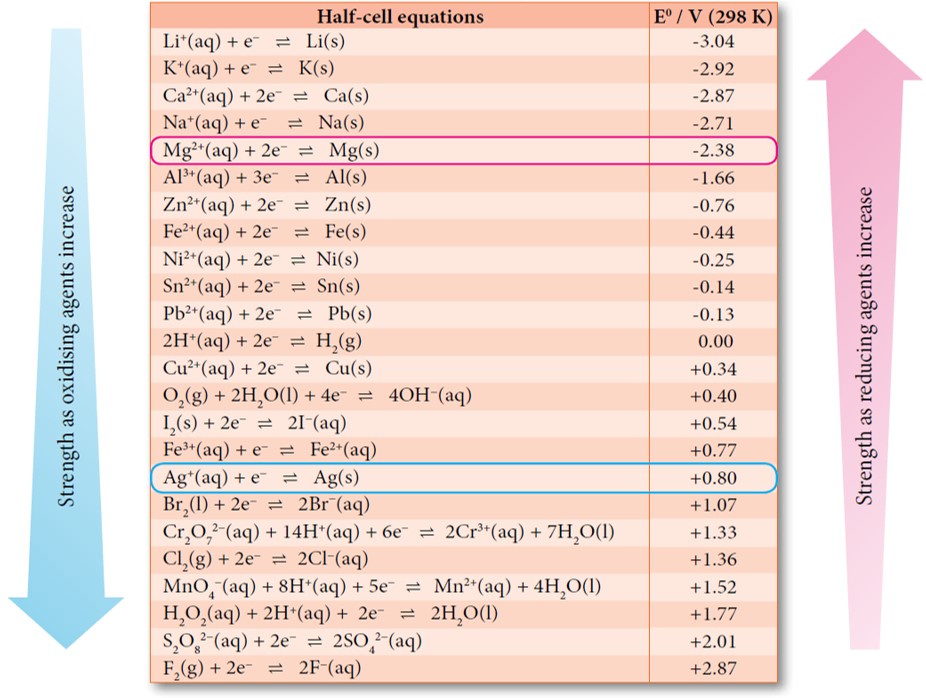
Using the standard electrode potentials given in the table, predict if the reaction between the following is possible. Ag^+(aq) and Cu(s)

Using the standard electrode potentials given in the table, predict if the reaction between the following is possible. Br2(aq) and Fe^2 + (aq)

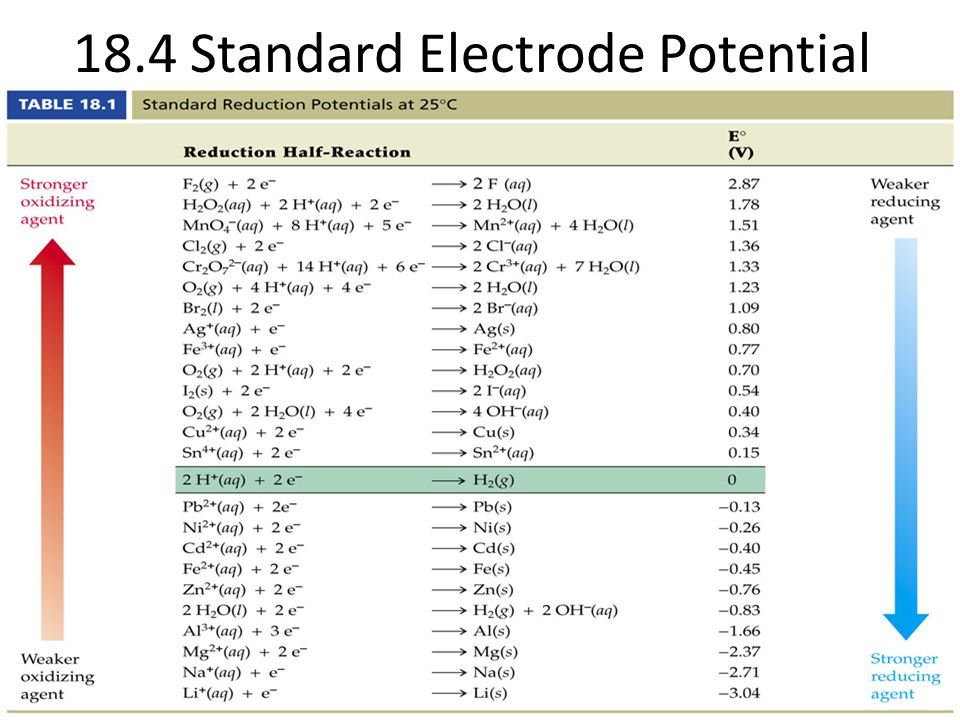
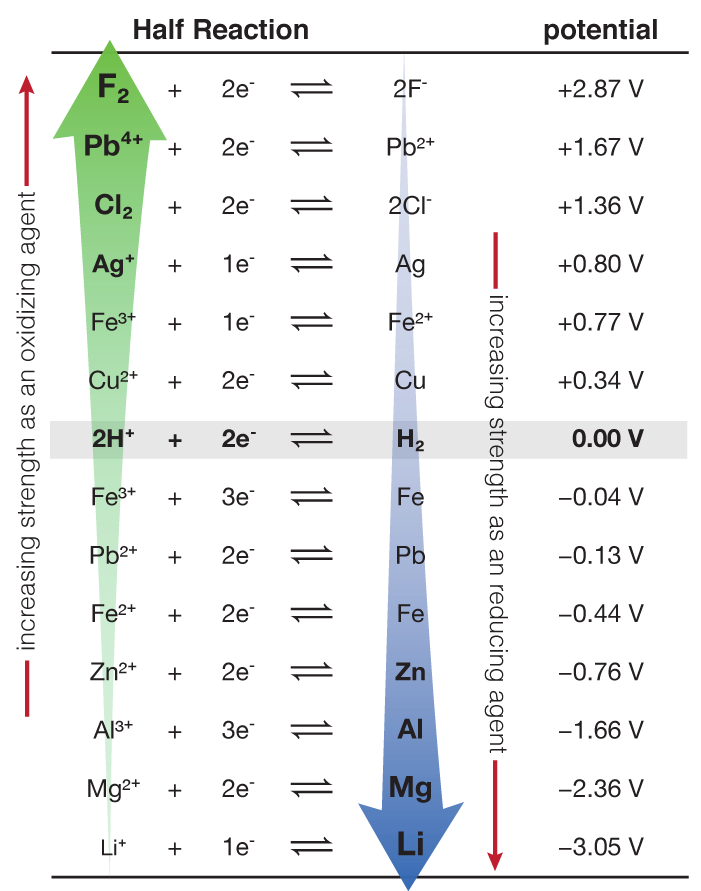
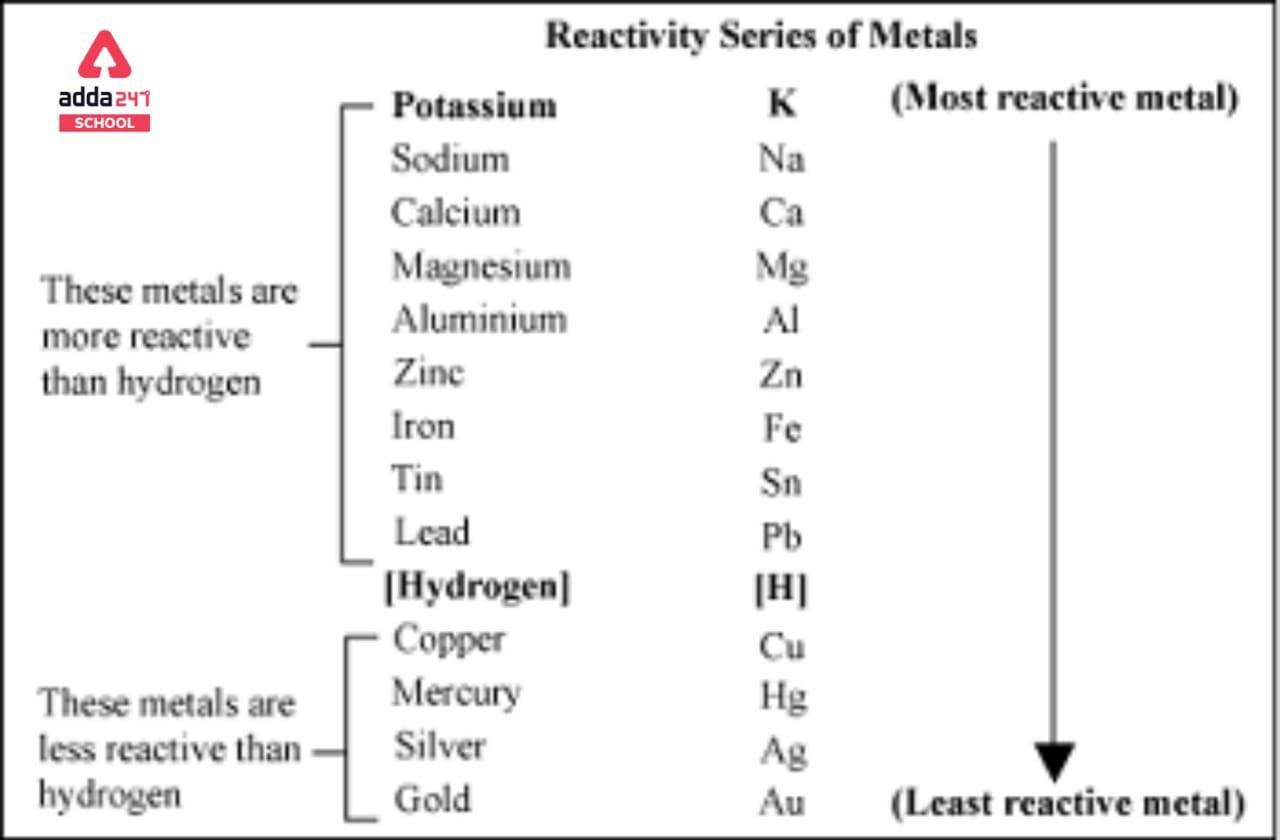
Table of selected standard electrode potentials in aqueous solutions at 25C | Solutions, Chemistry, Electrodes

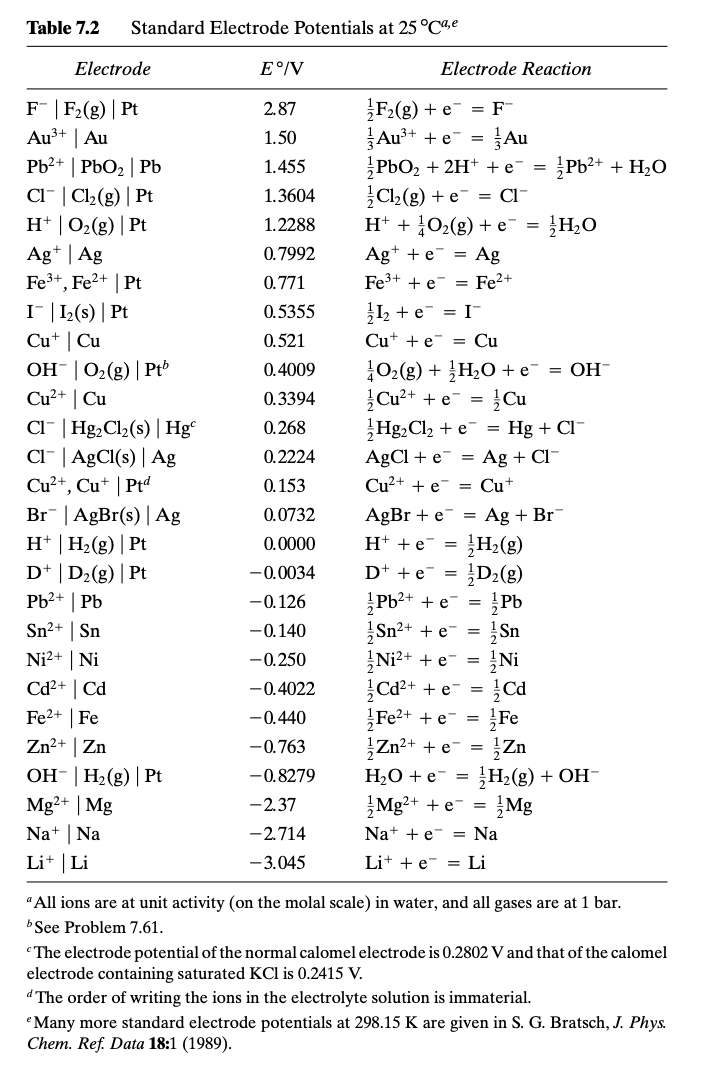
thermodynamics - Calculating the electrode potential from thermochemical data - Chemistry Stack Exchange
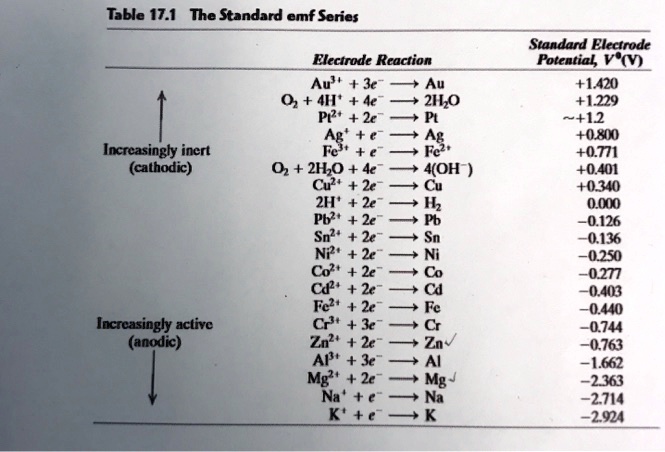
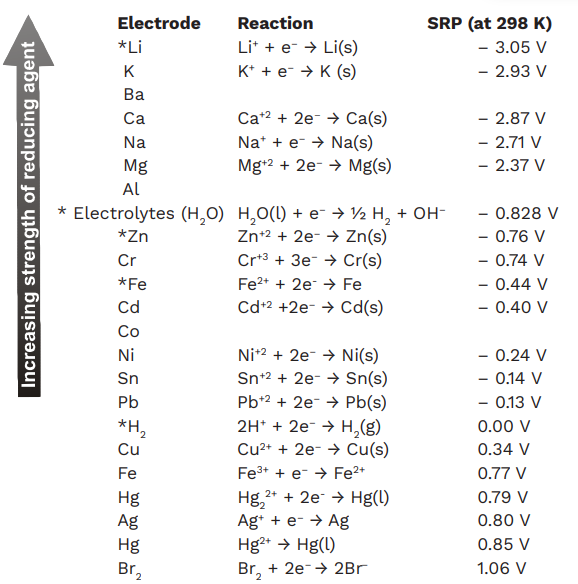
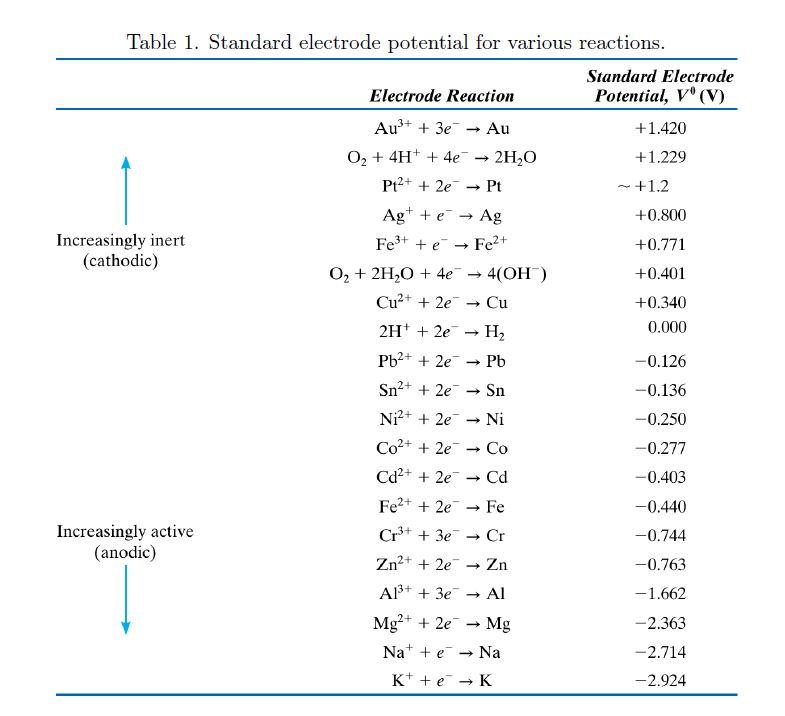
SOLVED: Table 12.1 The Standard emf Series Standard Electrode Potential V"(V) +1420 +1229 +12 +0800 +0.771 40.401 +0340 0.00 0.126 0.136 0.250 -0277 0.403 0.440 0.744 0.763 1.662 2363 -2714 -2924 Electrode